Research Projects
Genetic Engineering of T cells for Adoptive Immunotherapy of B-cell Malignancies
The extraordinary sensitivity and specificity of T cells for their cognate antigen make them a highly attractive cancer therapeutic. However, the rarity of tumor-reactive T cells in cancer patients, the difficulty isolating them in sufficient numbers for adoptive immunotherapy, and the unpredictable persistence of transferred cells have been significant obstacles to broad application. Technologies that enable genetic modification of T cells have been refined and are being used to redirect T cell specificity to tumor antigens and produce large numbers of potent tumor-reactive T cells. An important remaining issue is how the diverse phenotypic and functional heterogeneity in distinct T cell subsets can be capitalized upon to enhance the efficacy, safety and reproducibility of cancer immunotherapy (Figure 1).
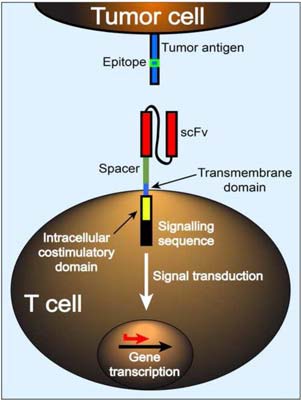
Dr. Turtle’s group developed a clinical platform for isolating functionally and phenotypically distinct subsets of T cells that could be subsequently genetically engineered to target CD19, which is expressed on the tumor cells in most patients with B-cell malignancies. The T cell subsets are re-directed to target CD19 by modifying them to express a “chimeric antigen receptor” (CAR), which binds to CD19 on the tumor surface.
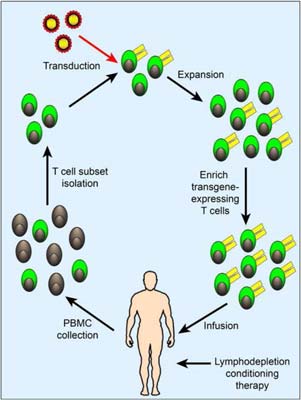
In these CAR-T cell immunotherapy trials, patients receive chemotherapy to make space in the immune system, followed by infusion of CAR-T cells (Figure 2). Afterwards, the patients are monitored closely to study the effects of the CAR-T cells. Detailed laboratory studies are ongoing in connection with the clinical trials, to monitor the function and phenotype of transferred T cells and to characterize features associated with distinct clinical outcomes.
CAR-T Cell Therapy of Acute Myeloid Leukemia
AML may be more difficult to treat with CAR-T cells than many B cell malignancies because of the lack of truly lineage-specific myeloid target antigens. We are developing new approaches to target AML using CAR-T cells.
Immune Reconstitution After HCT
Perturbed immune reconstitution after allogeneic HCT is an important factor that contributes to many post-HCT complications, such as tumor relapse and graft versus host disease (GVHD). We are studying the roles of subsets of non-conventional T cells, such as mucosal-associated invariant T cells (MAIT cells), Treg cells, iNKT cells and Th17 cells in post-HCT immune reconstitution and development of post-HCT complications. With FHCRC collaborators, we have a particular interest in investigating the role of the gastrointestinal microbiota in driving reconstitution of these subsets after HCT.

© 2025 Fred Hutchinson Cancer Center, a 501(c)(3) nonprofit organization.